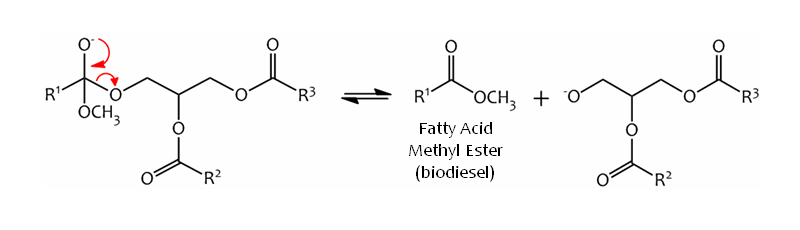Difference between revisions of "Biodiesel"
m (→Chemical process) |
(Move chemical process to the end of page; keeps the simple explaination and page flow together. Chemical process might need moving to its own page someday.) |
||
| Line 1: | Line 1: | ||
<metadesc>Biodiesel definition, Biodiesel description, Biodiesel brief introduction</metadesc> | <metadesc>Biodiesel definition, Biodiesel description, Biodiesel brief introduction</metadesc> | ||
| − | |||
| − | |||
Biodiesel (chemical name [http://en.wikipedia.org/wiki/Fatty_acid_methyl_esters Fatty Acid Methyl Esters]), has very similar combustion properties to mineral Diesel, making it a suitable substitute for mineral Diesel in road vehicles. | Biodiesel (chemical name [http://en.wikipedia.org/wiki/Fatty_acid_methyl_esters Fatty Acid Methyl Esters]), has very similar combustion properties to mineral Diesel, making it a suitable substitute for mineral Diesel in road vehicles. | ||
| Line 20: | Line 18: | ||
Biodiesel is made from vegetable oil using a chemical process to split the vegetable oil molecules. This is done in a biodiesel processor. | Biodiesel is made from vegetable oil using a chemical process to split the vegetable oil molecules. This is done in a biodiesel processor. | ||
| − | |||
| − | |||
Vegetable oil in molecular form looks similar to a garden hand fork with three prongs, some of which are bent and different lengths, and a very short, heavy handle. Imagine a large number of these in a bin. If you jiggle them all up (heating) and then let them settle (cooling) it's inevitable that when you go to pour them out of the bin, some will be tangled together (which is similar to how piston ring gumming with a cold engine occurs). | Vegetable oil in molecular form looks similar to a garden hand fork with three prongs, some of which are bent and different lengths, and a very short, heavy handle. Imagine a large number of these in a bin. If you jiggle them all up (heating) and then let them settle (cooling) it's inevitable that when you go to pour them out of the bin, some will be tangled together (which is similar to how piston ring gumming with a cold engine occurs). | ||
| Line 27: | Line 23: | ||
Converting vegetable oil into biodiesel is like breaking all the prongs off the forks and throwing the handles (glycerol) away. You can shake all the individual prongs up as much as you like but they won't tangle together as easily, which is why biodiesel doesn't gum up piston rings like vegetable oil. | Converting vegetable oil into biodiesel is like breaking all the prongs off the forks and throwing the handles (glycerol) away. You can shake all the individual prongs up as much as you like but they won't tangle together as easily, which is why biodiesel doesn't gum up piston rings like vegetable oil. | ||
| − | ===Chemical process | + | ==Blends== |
| + | |||
| + | B5, B30 and B100 refer to blends of biodiesel with mineral Diesel. B30 is 30% mineral Diesel, 70% Biodiesel. Some vehicles run happily on B100, whereas for others, the manufacturer may specify a maximum blend. Ambient temperature is also an influencing factor, as Biodiesel can "wax up" before mineral Diesel at lower temperatures. | ||
| + | |||
| + | ==Quality standards== | ||
| + | |||
| + | The European standard for Biodiesel is EN14214, although in the UK commercial producers are not legally required to produce fuel meeting this standard. Buyer beware. | ||
| + | |||
| + | In the US this standard is ASTM D 6751. | ||
| + | |||
| + | ==Chemical process== | ||
| − | Methanol is deprotonated using NaOH, to make it a stronger nucleophile:- | + | [[Methanol]] is deprotonated using [[NaOH]], to make it a stronger nucleophile:- |
[[File:Trans1.gif]] | [[File:Trans1.gif]] | ||
| − | The Methoxide attacks the electrophillic carbonyl carbon atom of the triglyceride via an Sn2 mechanism, forming a tetrahedral intermediate. | + | The [[Methoxide]] attacks the electrophillic carbonyl carbon atom of the triglyceride via an Sn2 mechanism, forming a tetrahedral intermediate. |
This is an equilibrium reaction so an excess of Methanol is used to drive the equilibrium to the right, favouring the formation of product. | This is an equilibrium reaction so an excess of Methanol is used to drive the equilibrium to the right, favouring the formation of product. | ||
| Line 51: | Line 57: | ||
''(special thanks to Twenty4Seven for the images and explanatory chemistry)'' | ''(special thanks to Twenty4Seven for the images and explanatory chemistry)'' | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Biodiesel]] | [[Category:Biodiesel]] | ||
[[Category:Introduction to Biodiesel]] | [[Category:Introduction to Biodiesel]] | ||
Revision as of 15:18, 17 June 2011
Biodiesel (chemical name Fatty Acid Methyl Esters), has very similar combustion properties to mineral Diesel, making it a suitable substitute for mineral Diesel in road vehicles.
Biodiesel should not be confused with vegetable oil, or blends of vegetable oil with solvents such as Petrol.
Advantages
Biodiesel presents several advantages over pure vegetable oil as a road fuel:
- significantly reduced viscosity; good for systems with weak mechanical injection pumps (EG Lucas, which can shear its driveshaft with vegetable oil)
- lower melt point; can be used in winter
- can be safely cold-started; no piston ring gumming and resulting loss of compression
How it is made
Biodiesel is made from vegetable oil using a chemical process to split the vegetable oil molecules. This is done in a biodiesel processor.
Vegetable oil in molecular form looks similar to a garden hand fork with three prongs, some of which are bent and different lengths, and a very short, heavy handle. Imagine a large number of these in a bin. If you jiggle them all up (heating) and then let them settle (cooling) it's inevitable that when you go to pour them out of the bin, some will be tangled together (which is similar to how piston ring gumming with a cold engine occurs).
Converting vegetable oil into biodiesel is like breaking all the prongs off the forks and throwing the handles (glycerol) away. You can shake all the individual prongs up as much as you like but they won't tangle together as easily, which is why biodiesel doesn't gum up piston rings like vegetable oil.
Blends
B5, B30 and B100 refer to blends of biodiesel with mineral Diesel. B30 is 30% mineral Diesel, 70% Biodiesel. Some vehicles run happily on B100, whereas for others, the manufacturer may specify a maximum blend. Ambient temperature is also an influencing factor, as Biodiesel can "wax up" before mineral Diesel at lower temperatures.
Quality standards
The European standard for Biodiesel is EN14214, although in the UK commercial producers are not legally required to produce fuel meeting this standard. Buyer beware.
In the US this standard is ASTM D 6751.
Chemical process
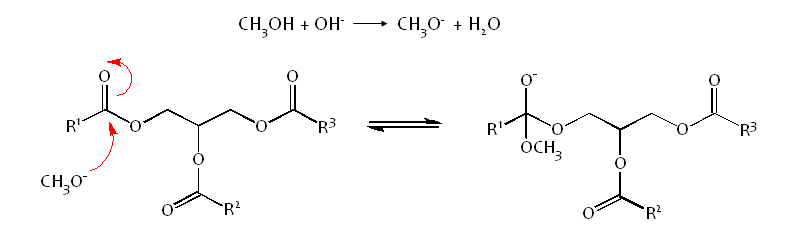
Methanol is deprotonated using NaOH, to make it a stronger nucleophile:-
The Methoxide attacks the electrophillic carbonyl carbon atom of the triglyceride via an Sn2 mechanism, forming a tetrahedral intermediate.
This is an equilibrium reaction so an excess of Methanol is used to drive the equilibrium to the right, favouring the formation of product.
The tetrahedral intermediate collapses and expels the alkoxy anion of the diglyceride, leaving the Methyl Ester product:-
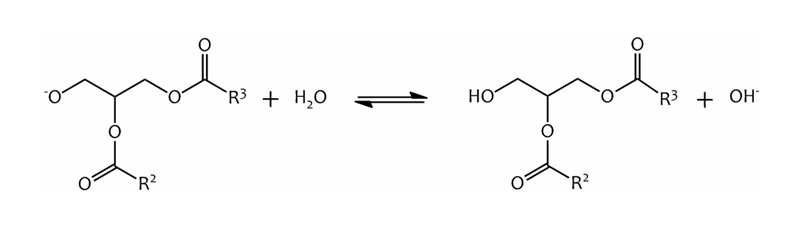
Finally, the diglyceride anion is protonated, regenerating the catalyst:-
The regenerated catalyst is now able to react with another Methanol molecule, beginning another cycle.
The transesterification continues until all three fatty acid groups have been converted to Methyl Esters, leaving just the Glycerol backbone as the main by-product.
(special thanks to Twenty4Seven for the images and explanatory chemistry)