Difference between revisions of "Titration"
| Line 2: | Line 2: | ||
[[File:Turmeric.jpg|250px|thumb|right|Various stages of the titration test.]] | [[File:Turmeric.jpg|250px|thumb|right|Various stages of the titration test.]] | ||
[[File:25ml burette.jpg|250px|thumb|right|Laboratory standard titration equipment. By NigelB]] | [[File:25ml burette.jpg|250px|thumb|right|Laboratory standard titration equipment. By NigelB]] | ||
| − | If you make biodiesel from fresh, unused vegetable oil there is no need to titrate the oil. This is because unused oil contains little or no [[ | + | If you make biodiesel from fresh, unused vegetable oil there is no need to titrate the oil. This is because unused oil contains little or no [[free fatty acid]]s, this allows base weights of (5 grams per litre of oil for [[NaOH]] and 7 grams per litre for [[KOH]]) to be used in the biodiesel reaction. |
| − | Used vegetable oil will contain | + | Used vegetable oil will contain free fatty acids in varying amounts depending on how, and for how long, it has been used. |
Free fatty acids play no part in the biodiesel reaction and need to be removed. This is done by adding additional catalyst with which it will react to form soap. The soap is later removed as part of the production process. | Free fatty acids play no part in the biodiesel reaction and need to be removed. This is done by adding additional catalyst with which it will react to form soap. The soap is later removed as part of the production process. | ||
| Line 68: | Line 68: | ||
===pH indicator solution=== | ===pH indicator solution=== | ||
| − | See also [[Titrating with | + | See also: [[Titrating with turmeric]] |
| − | Indicators are substances that change colour at a certain pH. All indicator solutions are made by adding a little bit of an indicator to a lot of alcohol. They change colour when the solution they are in changes to a specific pH level. The two most common indicators used for making biodiesel are [http://en.wikipedia.org/wiki/Phenolphthalein | + | Indicators are substances that change colour at a certain pH. All indicator solutions are made by adding a little bit of an indicator to a lot of alcohol. They change colour when the solution they are in changes to a specific pH level. The two most common indicators used for making biodiesel are [http://en.wikipedia.org/wiki/Phenolphthalein phenolphthalein], and [[Titrating with Turmeric|turmeric]]. |
Phenolphthalein is the industry standard for titrating fats and oils to determine the percentage of free fatty acids they contain. It must be handled with care because it is an irritant. Phenolphthalein changes from colourless to pink at a pH 8.2 | Phenolphthalein is the industry standard for titrating fats and oils to determine the percentage of free fatty acids they contain. It must be handled with care because it is an irritant. Phenolphthalein changes from colourless to pink at a pH 8.2 | ||
| Line 78: | Line 78: | ||
As turmeric is safe and readily available, this page will concentrate on its use. It needs to be made into a solution as follows: | As turmeric is safe and readily available, this page will concentrate on its use. It needs to be made into a solution as follows: | ||
| − | In a clean jar mix | + | In a clean jar mix isopropyl alcohol and powdered turmeric in the ratio of 5 parts isopropyl alcohol to 1 part turmeric by volume and mix well. The proportions are not too critical, but you should end up with a bright yellow solution with the turmeric powder settled at the bottom. Decant off the liquid into your dropper bottle and dispose of the dregs. For each titration you will only use a few drops so you don’t need to make up a massive quantity. As the rate of use is slow it is advisable to make fresh solution every few months to ensure that you always obtain accurate results. |
Turmeric solution is obviously non hazardous and non toxic but it will severely stain clothes and work surfaces so take suitable precautions. | Turmeric solution is obviously non hazardous and non toxic but it will severely stain clothes and work surfaces so take suitable precautions. | ||
| Line 92: | Line 92: | ||
Both the raw catalyst and the solution you have made are hygroscopic. This means they will absorb water from the atmosphere. If this happens to any large extent it will render the raw catalyst unusable and the reference solution inaccurate so keep both in air tight containers and only expose to the air for as short a time as possible. | Both the raw catalyst and the solution you have made are hygroscopic. This means they will absorb water from the atmosphere. If this happens to any large extent it will render the raw catalyst unusable and the reference solution inaccurate so keep both in air tight containers and only expose to the air for as short a time as possible. | ||
| − | For each titration you will only require a very small quantity of reference solution, typically a maximum of 10ml, so a one litre batch will give you at least 100 tests depending on the titration level of your oil. As with the indicator solution it is best practice to make a fresh solution after a few months to ensure the accuracy of the test. Similarly, if you purchase a new supply of catalyst for | + | For each titration you will only require a very small quantity of reference solution, typically a maximum of 10ml, so a one litre batch will give you at least 100 tests depending on the titration level of your oil. As with the indicator solution it is best practice to make a fresh solution after a few months to ensure the accuracy of the test. Similarly, if you purchase a new supply of catalyst for biodiesel production, then you should make a new reference solution using the new catalyst. |
==The titration process== | ==The titration process== | ||
| Line 104: | Line 104: | ||
===Titration overview=== | ===Titration overview=== | ||
| − | The object of the test is to ascertain how much additional catalyst will be required to convert the | + | The object of the test is to ascertain how much additional catalyst will be required to convert the free fatty acids to soap. This is done by dissolving oil in [[isopropyl Alcohol]], adding indicator solution and then slowly adding the reference solution until the pH is brought to 8.5, at which point the indicator will change colour. As we are looking for quite small changes in pH levels it is imperative that all equipment is thoroughly clean before proceeding so as not to influence the result. |
===Blank titration=== | ===Blank titration=== | ||
| − | For accurate results a blank titration should be carried out. This is a process to ensure that the | + | For accurate results a blank titration should be carried out. This is a process to ensure that the isopropyl alcohol if slightly acidic, will be neutralised and therefore not effect the accuracy of the titration. |
| − | Measure out 10ml of | + | Measure out 10ml of isopropyl alcohol in a jar or beaker. Add a few drops of pH indicator solution and ensure it’s thoroughly mixed. Take 2 or 3 ml of reference solution in a pipette or syringe and slowly add it to the jar drop by drop whilst stirring continuously. When the isopropyl alcohol reaches a pH of 8.5 the indicator solution will change colour. Turmeric will change from bright yellow to a dark crimson and phenolphthalein will change from yellow to pink. |
| − | If the colour change remains for 10 to 15 seconds, you have added enough reference solution and the | + | If the colour change remains for 10 to 15 seconds, you have added enough reference solution and the isopropyl alcohol is ready for use. |
<gallery caption="Blank titration sequence" widths="200px" heights="200px" perrow="3"> | <gallery caption="Blank titration sequence" widths="200px" heights="200px" perrow="3"> | ||
| − | File:Blank titration 1.jpg|10 ml | + | File:Blank titration 1.jpg|10 ml isopropyl alcohol with a few drops of turmeric indicator solution. |
File:Blank titration 2.jpg|With a few drops of reference solution added, on the point of colour change. | File:Blank titration 2.jpg|With a few drops of reference solution added, on the point of colour change. | ||
File:Blank titration 3.jpg|With one more drop of reference solution, complete colour chance and ready for titration. | File:Blank titration 3.jpg|With one more drop of reference solution, complete colour chance and ready for titration. | ||
| Line 124: | Line 124: | ||
===Titration=== | ===Titration=== | ||
| − | Draw 1ml of oil into a clean pipette or syringe, add to the | + | Draw 1ml of oil into a clean pipette or syringe, add to the isopropyl alcohol and mix thoroughly. Draw a known volume of reference solution into a clean pipette or syringe, let’s say 5 ml. As with the blank titration add this to the isopropyl alcohol/oil mix drop by drop, stirring continuously until the indicator changes colour. If the colour change is stable for 20-30 seconds or so the titration is complete. |
Note how much reference solution has been used. So if you have 2ml left in the pipette you have used 3ml and the titration value of the oil is 3. | Note how much reference solution has been used. So if you have 2ml left in the pipette you have used 3ml and the titration value of the oil is 3. | ||
| Line 134: | Line 134: | ||
<gallery caption="Titration sequence" widths="200px" heights="200px" perrow="3"> | <gallery caption="Titration sequence" widths="200px" heights="200px" perrow="3"> | ||
| − | File:Titration 1.jpg|Blank titrated | + | File:Titration 1.jpg|Blank titrated isopropyl alcohol with 1ml of oil added note the cloudy appearance and the colour change back to yellow. |
File:Titration 2.jpg|With a few drops of reference solution added, colour change has started. | File:Titration 2.jpg|With a few drops of reference solution added, colour change has started. | ||
File:Titration 3.jpg|Additional reference solution added and colour change is complete. | File:Titration 3.jpg|Additional reference solution added and colour change is complete. | ||
| Line 148: | Line 148: | ||
7÷0.95 = 7.4 g | 7÷0.95 = 7.4 g | ||
| − | Conveniently the titration value equates to the number of grams per litre required to neutralise the | + | Conveniently the titration value equates to the number of grams per litre required to neutralise the free fatty acids. |
| Line 184: | Line 184: | ||
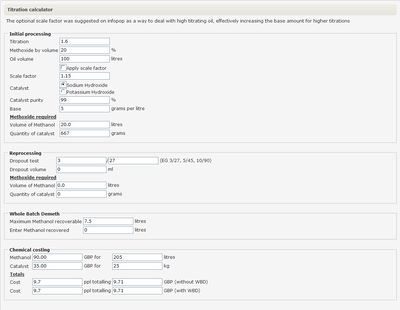
An alternative to the above calculations is offered by Tony in the form of a comprehensive [http://www.biopowered.co.uk/forum/tools/titration.php Titration calculator.] | An alternative to the above calculations is offered by Tony in the form of a comprehensive [http://www.biopowered.co.uk/forum/tools/titration.php Titration calculator.] | ||
| − | This form will not only calculate the amount of catalyst to be used, but also | + | This form will not only calculate the amount of catalyst to be used, but also methanol and catalyst requirements for re-processing, recoverable methanol when using [[Whole batch demeth|WBD]] and the cost of producing the batch. |
<br> | <br> | ||
Latest revision as of 18:06, 27 February 2013
If you make biodiesel from fresh, unused vegetable oil there is no need to titrate the oil. This is because unused oil contains little or no free fatty acids, this allows base weights of (5 grams per litre of oil for NaOH and 7 grams per litre for KOH) to be used in the biodiesel reaction.
Used vegetable oil will contain free fatty acids in varying amounts depending on how, and for how long, it has been used.
Free fatty acids play no part in the biodiesel reaction and need to be removed. This is done by adding additional catalyst with which it will react to form soap. The soap is later removed as part of the production process.
Titration is a test that determines the level of free fatty acids known as the acid value or acid number.
It is very important to obtain accurate titration results, as these will have a major bearing on the quality of the finished biodiesel. For best accuracy, two or more titration tests can be carried out and the results averaged, especially if the titration levels are high. Experience has shown that pH meters don't work with WVO and are no substitute for a simple titration using indicator solution.
Contents
Equipment required
The items required for the test are very basic, although you can go to the expense of laboratory equipment, most home brewers don’t bother. Keep an eye on the household rubbish, over time most bits and pieces required can be found and reused. As we are private individuals carrying out this process at home we are not obliged to label any storage bottles to the same degree as would be required in a commercial situation. It is however, good practice and very strongly recommended, to clearly and indelibly mark each storage container with its contents and for solutions you make up, add the date so you have an idea of when they may need replacing.
Scales
In the past, home brewers have advocated making balances with which to weigh out chemicals. However, very accurate digital scales are now available at extremely reasonable prices via the internet and advertised as jewelry scales, it is these that are now pretty much standard equipment in the home bio lab.
The scales will need to have a minimum resolution of 0.1 grams. You will only need this degree of accuracy infrequently for making your titration reference solution. However, if the range of the scales is large enough, say up to 1Kg, they can also be used for weighing the catalyst during biodiesel production which saves buying two sets of scales, although with large processors and high titrating oil it may be necessary to weigh the catalyst in two hits.
The platen on this type of scale is small but it’s capacity can be increased by using a suitable jug to contain the chemical and then using the “tare” facility to reset zero.
Beakers
Beakers in the laboratory, but jars in the bio lab. You will require a minimum of two jars. One to hold the oil sample, a jam jar is perfect for this and a smaller jar for the titration reaction. A baby food or fish paste jar is more suitable for this operation. Although two is a minimum, it’s always handy to have several jars to hand for spent solutions and used syringes and pipettes etc.
Measuring cylinders
As with beakers laboratory type measuring cylinders can be used but plastic measuring cups form the kitchen work equally as well. For titrations only small volumes of 10ml are required, so a medicine measure might be employed or a syringe, see below. For making reference solutions you will need to measure 1 litre accurately and this will be done more conveniently with a larger vessel.
Syringes and pipettes
It’s largely down to preference as to which you use, but you will need two, possibly three sizes. A 20ml syringe will be useful if you don’t use the 10ml measuring cup mentioned above and something like 3ml plastic pipettes for measuring the oil sample and reference solution. If you have high titrating oil, a syringe of around 10ml may be more convenient.
Personally I prefer to use the plastic, disposable pipettes, they are cheap to buy and are far easier to use with one hand than a syringe.
Dropper bottle
A small dropper bottle will be needed to store and dispense pH indicator solution. One similar to this is perfect, but a glass “eye dropper” bottle could equally be employed.
Reference solution storage bottle
This can be a glass or plastic bottle of between 500 ml and 1 litre. If using plastic HDPE is recommended as the reference solution is a mild base. I use an old gear oil bottle which has a translucent strip moulded into one side allowing the contents level to be viewed. What ever you use the top must be air tight to stop the solution coming into contact with the air.
Chemicals required
The following chemicals will be required for the titration process. All can be purchased via the internet and some from supermarkets or hardware stores. Be sure that you obtain a Material Safety Data Sheet (MSDS) for each product you buy. It is a legal obligation on the part of the seller to supply this. Ask before purchasing, if an MSDS is not supplied, find another supplier.
Isopropyl alcohol
1 litre should be sufficient for 100 titrations. Aim to buy the most pure you can at least 95% or better.
pH indicator solution
See also: Titrating with turmeric
Indicators are substances that change colour at a certain pH. All indicator solutions are made by adding a little bit of an indicator to a lot of alcohol. They change colour when the solution they are in changes to a specific pH level. The two most common indicators used for making biodiesel are phenolphthalein, and turmeric.
Phenolphthalein is the industry standard for titrating fats and oils to determine the percentage of free fatty acids they contain. It must be handled with care because it is an irritant. Phenolphthalein changes from colourless to pink at a pH 8.2
Turmeric is by no means new as an indicator and references to it can be found in chemistry texts. Because turmeric is readily available in the spice section of most supermarkets, is cheap and lacks the irritant effect of phenolphthalein, its use as an indicator is preferred by the majority of home biodiesel makers. Turmeric changes colour from yellow to orange/red at a pH 8.5, close to that of phenolphthalein.
As turmeric is safe and readily available, this page will concentrate on its use. It needs to be made into a solution as follows:
In a clean jar mix isopropyl alcohol and powdered turmeric in the ratio of 5 parts isopropyl alcohol to 1 part turmeric by volume and mix well. The proportions are not too critical, but you should end up with a bright yellow solution with the turmeric powder settled at the bottom. Decant off the liquid into your dropper bottle and dispose of the dregs. For each titration you will only use a few drops so you don’t need to make up a massive quantity. As the rate of use is slow it is advisable to make fresh solution every few months to ensure that you always obtain accurate results. Turmeric solution is obviously non hazardous and non toxic but it will severely stain clothes and work surfaces so take suitable precautions.
Reference solution
This needs to be a very accurately made solution of your catalyst, be it NaOH or KOH and distilled or deionised water.
Using clean equipment accurately measure out 1 litre of distilled water. Weigh out 1 gram of catalyst to an accuracy of 0.1gram (this is why you require scales of a similar accuracy). It is best practice to use catalyst from the same batch as you will use to make your biodiesel. Ideally the catalyst should be 99% pure. Add the catalyst to the water and stir carefully until completely dissolved.
A more accurate method of mixing a reference solution is to use the procedure above but use 10 grams of catalyst to 1 litre of distilled water. Then take 100ml of this solution and mix with a further 900ml of distilled water.
Both the raw catalyst and the solution you have made are hygroscopic. This means they will absorb water from the atmosphere. If this happens to any large extent it will render the raw catalyst unusable and the reference solution inaccurate so keep both in air tight containers and only expose to the air for as short a time as possible.
For each titration you will only require a very small quantity of reference solution, typically a maximum of 10ml, so a one litre batch will give you at least 100 tests depending on the titration level of your oil. As with the indicator solution it is best practice to make a fresh solution after a few months to ensure the accuracy of the test. Similarly, if you purchase a new supply of catalyst for biodiesel production, then you should make a new reference solution using the new catalyst.
The titration process
Preparation
Safety first. To carry out titration tests you should wear rubber or latex gloves, long sleeves and suitable eye protection. Assemble all the equipment and chemicals on a clear, stable work surface and be clear in you mind as to how you are going to proceed.
Take a small sample of oil. The sample should obviously be from the batch of oil you are about to convert to biodiesel. As most people collect oil in small batches from different sources and combine them to make one batch, it is preferable to have previously loaded the oil into the processor and mixed it thoroughly whilst heating. This ensures two things, firstly that the oil is a homogeneous and representative sample of the whole batch and secondly that it is not too cold to carry out the titration. For accurate results titrations should be carried out at approximately 20°C, having the oil pre-warmed will assist.
Titration overview
The object of the test is to ascertain how much additional catalyst will be required to convert the free fatty acids to soap. This is done by dissolving oil in isopropyl Alcohol, adding indicator solution and then slowly adding the reference solution until the pH is brought to 8.5, at which point the indicator will change colour. As we are looking for quite small changes in pH levels it is imperative that all equipment is thoroughly clean before proceeding so as not to influence the result.
Blank titration
For accurate results a blank titration should be carried out. This is a process to ensure that the isopropyl alcohol if slightly acidic, will be neutralised and therefore not effect the accuracy of the titration.
Measure out 10ml of isopropyl alcohol in a jar or beaker. Add a few drops of pH indicator solution and ensure it’s thoroughly mixed. Take 2 or 3 ml of reference solution in a pipette or syringe and slowly add it to the jar drop by drop whilst stirring continuously. When the isopropyl alcohol reaches a pH of 8.5 the indicator solution will change colour. Turmeric will change from bright yellow to a dark crimson and phenolphthalein will change from yellow to pink.
If the colour change remains for 10 to 15 seconds, you have added enough reference solution and the isopropyl alcohol is ready for use.
- Blank titration sequence
Titration
Draw 1ml of oil into a clean pipette or syringe, add to the isopropyl alcohol and mix thoroughly. Draw a known volume of reference solution into a clean pipette or syringe, let’s say 5 ml. As with the blank titration add this to the isopropyl alcohol/oil mix drop by drop, stirring continuously until the indicator changes colour. If the colour change is stable for 20-30 seconds or so the titration is complete.
Note how much reference solution has been used. So if you have 2ml left in the pipette you have used 3ml and the titration value of the oil is 3.
It is good practice to repeat the process to ensure accuracy. You should expect the second result to be within 0.2 – 0.3ml of the first. The two values can then be averaged and this result taken as the titration value.
If the results vary significantly there has most likely been contamination during the titration and the tests should be re-run with clean equipment.
- Titration sequence
Using the results of a titration test
Knowing the titration value we can now calculate the total weight of catalyst required for the batch of biodiesel. As mentioned at the top of the page the weight of catalyst required to convert new oil is a known value, 5 grams per litre for NaOH and 7 grams per litre for KOH. If you are just starting out with biodiesel production it’s safest to stick to these weights, but by varying the production process and by experimentation many home brewers have found these figures can be considerably reduced.
If your catalyst has a low purity often the case with KOH, it’s good idea to make an adjustment to the base figure to compensate for this.
Therefore if your KOH is 95% pure, the base weight should be increased as follows: 7÷0.95 = 7.4 g
Conveniently the titration value equates to the number of grams per litre required to neutralise the free fatty acids.
We’ll stick with the generally accepted base weight figures for these examples.
Three pieces of information are needed for the calculation:
- The volume of oil to be processed … say 100 litres.
- The type of catalyst being used … say NaOH giving a base weight of 5 grams per litre
- And the titration value … from the above example, 3 i.e. 3 grams
Therefore the total weight of catalyst required will be the base weight (5) plus the weight derived from the titration (3) times the number of litres of oil to be processed (100), so:
(5+3)×100 = 800 grams.
Another example using KOH at 95% purity.
- The volume of oil to be processed … say 80 litres.
- The type of catalyst being used … say 95% KOH adjusted to compensate for purity, giving a base weight of 7.4 grams per litre
- And the titration value … say, 0.5 i.e. 0.5 grams per litre
Therefore the total weight of catalyst required will be the base weight (7 adjusted to compensate for purity) plus the weight derived from the titration (0.5) times the number of litres of oil to be processed (80), so:
((7÷0.95)+0.5)×80 = 632 grams.
Titration calculator
An alternative to the above calculations is offered by Tony in the form of a comprehensive Titration calculator.
This form will not only calculate the amount of catalyst to be used, but also methanol and catalyst requirements for re-processing, recoverable methanol when using WBD and the cost of producing the batch.
See also
Bio-rich-time-poor 18:44, 28 November 2010 (UTC)