Difference between revisions of "Titrating with turmeric"
| Line 1: | Line 1: | ||
| − | == | + | ==Introduction== |
| − | When making our own biodiesel out of waste vegetable oil (WVO), we need to know how much to adjust the amount of NaOH/KOH required to neutralize the free fatty acids (FFAs) the wvo contains. We determine the percentage of FFAs in a given sample of wvo by using an indicator solution to titrate it. The titration gives us the information we need to calculate the amount of extra NaoH/KOH it will take to neutralize the FFAs. | + | When making our own biodiesel out of waste vegetable oil (WVO), we need to know how much to adjust the amount of NaOH/KOH catalyst required to neutralize the free fatty acids (FFAs) the wvo contains. We determine the percentage of FFAs in a given sample of wvo by using an indicator solution to titrate it. The titration gives us the information we need to calculate the amount of extra NaoH/KOH it will take to neutralize the FFAs. |
| − | |||
| + | _TOC_ | ||
| Line 99: | Line 99: | ||
'''PLEASE NOTE''' | '''PLEASE NOTE''' | ||
Turmeric has a very strong yellow colouring and can seriously stain other materials it comes into contact with. | Turmeric has a very strong yellow colouring and can seriously stain other materials it comes into contact with. | ||
| + | |||
Turmeric titration colour changes | Turmeric titration colour changes | ||
[[File:Turmeric.jpg|center|Turmeric titration colour changes]] | [[File:Turmeric.jpg|center|Turmeric titration colour changes]] | ||
Revision as of 11:32, 27 November 2010
Contents
Introduction
When making our own biodiesel out of waste vegetable oil (WVO), we need to know how much to adjust the amount of NaOH/KOH catalyst required to neutralize the free fatty acids (FFAs) the wvo contains. We determine the percentage of FFAs in a given sample of wvo by using an indicator solution to titrate it. The titration gives us the information we need to calculate the amount of extra NaoH/KOH it will take to neutralize the FFAs.
_TOC_
Why We Use Indicators to Titrate
When titrating WVO to make biodiesel, we use an indicator solution to tell us when the FFAs have been neutralized. Experience has shown that pH meters don't work with wvo and are no substitute for a simple titration using indicator solution.
All About Indicators
Indicators are substances that change color at a certain pH. All indicator solutions are made by adding a little bit of an indicator to a lot of alcohol. They do not change color until the solution they are in becomes acidic or alkaline. The three most common indicators used for making biodiesel are phenolphthalein, phenol red, and turmeric. Phenolphthalein and turmeric change color at the correct pH but phenol red does not.
Phenolphthalein
this is the industry standard for titrating fats and oils to determine the percentage of FFAs they contain. It must be handled with care because it is toxic. Phenolphthalein changes from colorless to pink. Turmeric is by no means new as an indicator and references to it can be found in chemistry texts. Because turmeric is readily available in the spice section of many grocery stores and lacks the toxicity of phenolphthalein, its use as an indicator is on the rise.
Turmeric
this changes color from yellow to orange/red at a pH close to that of phenolphthalein. Until recently, phenol red was frequently utilized for biodiesel titrations because it is available in countries where there are pool or spa shops. But because it changes color at a more acidic pH, experience has shown that phenol red is not suitable for use in biodiesel titrations unless the oil is known to titrate below 3.
What Is a "Blank Titration" and Why Do I Care?
The alcohol used to make indicator solutions may itself be acidic. This may also be true of the isopropyl alcohol used as a solvent in the titration itself. So it is common practice to do a "Blank Titration" to neutralize any acid which might be in the alcohol itself.
When you perform a "Blank Titration" you add titration indicator solution to alcohol and then add NaOH/KOH tester solution one drop at a time. Once the indicator just starts to turn color in the blank titration you know all of the acid in the alcohol has been neutralized or eliminated.
After you neutralize the acid in the alcohol, you know that the titration will measure just the acid in the oil itself. Following a "Blank Titration", you can simply add the oil to the neutralized alcohol and proceed to perform the titration itself.
Turmeric indicator solution starts to change color between pH 7.4 (above neutral) and pH 8.5 (alkaline). For our purposes as biodiesel homebrewers, this is accurate enough. With some alcohol, adding just one or two drops of NaOH/KOH tester solution to a "Blank Titration" may achieve a color change but with other alcohol it may take seven or eight drops.
What Does It Mean to "Neutralize" Free Fatty Acids (FFAs) to pH 8.5
When we titrate oil in solution with alcohol, we conduct a chemical reaction between the FFAs in the oil and the NaOH or KOH in the solution. This chemical reaction makes soap, which is alkaline.
Chemists have figured out that when ALL the FFAs have been turned into soap, the pH of the solution will rise to pH 8.5. They refer to this process as neutralization, as the FFAs (acids) have been eliminated. But keep in mind that this neutralized solution is alkaline (pH 8.5), NOT chemically neutral (pH 7).
Indicators that can tell us when a solution has a pH around pH 8.5 are most appropriate for biodiesel homebrewers. That is why turmeric and phenolphthalein are suitable, as they change color at pH levels that are close enough to pH 8.5 for our purposes. Turmeric contains a yellow pigment called curcumin that is yellow at pH 7.4 and orange to red at pH 8.6. Phenolpthalein is colorless up to pH 8.3 when it turns pink, through to deep red at pH 9.
Chemists before us figured out what a titration's pH is supposed to be and the experiences of thousands of biodiesel homebrewers have confirmed this in practice. So if you use an indicator (pH strips, bromthymol blue, etc.) that requires you to compare the color of your results against a scale, be sure that you titrate to pH 8.5 NOT to pH 7.
Indicator Color Change Persists 30 Seconds During Titrations
Whether you are performing a "Blank Titration" of alcohol or titrating oil in an alcohol solution, look for a color change that persists for 30 seconds. The exact color or shade is relatively unimportant so long as it:
IS NOT the same color as when you began the titration
IS the same shade when you're doing a "Blank Titration" (which will be transparent) AND when you're titrating oil (which will be duller or translucent)
So if one person's turmeric indicator starts yellow and changes into an orange-rose and someone else's starts greenish-yellow and changes into orange or red (as in this example picture at the bottom of the page), it doesn't matter. What matters is that the color has changed from the original yellow color to the same colour as your blank titration and stayed that way for 30 seconds, do some tests with your turmeric and observe the shade or colour it actually changes to when blanked.
This means that you shouldn't "push" a turmeric or phenolphthalein titration to yield a particular shade or intensity of color. As soon as the color changes and persists for 30 seconds, the titration is complete. The key is to use a consistent approach from titration to titration, it should be noted that when titrating with turmeric the titration mixture has a cloudy look to it and is not clear like phenolpthalien.
How to titrate with Turmeric
First, make a 0.1% NaOH/KOH tester solution- 1 gram of catalyst (NaOH or KOH) dissolved in 1 litre of distilled water as per:- Step 1: Make Reference Tester Solution on the titration page
Making a Turmeric Indicator Solution
1. Thoroughly mix ground turmeric with isopropyl alcohol in the following proportions:
1 part turmeric to 5 parts alcohol
2. Let the mixture settle
3. Decant the liquid into a small bottle with a dropper.
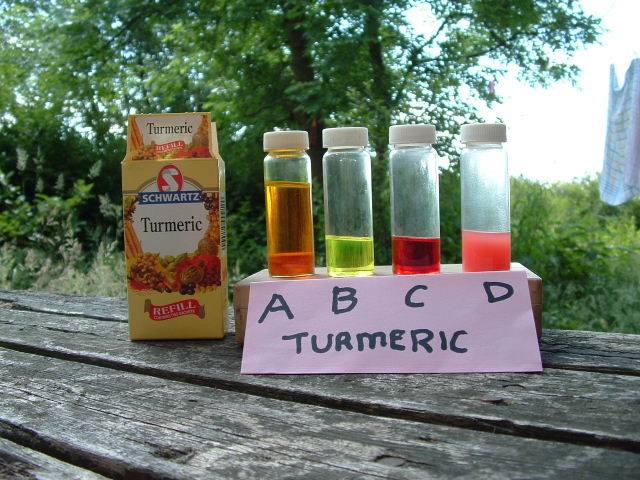
see picture below sample A
Performing a "Blank Titration"
1. Measure 10 ml. isopropyl alcohol into your titration vessel.
2. Add several drops of turmeric indicator solution until the alcohol turns yellow.
see picture below sample B
3. Add just enough drops of NaOH/KOH tester solution until you see a 30 second red color change.
see picture below sample C
How to titrate WVO
1. To the 10 ml of isopropyl alcohol that you have neutralized in a "Blank Titration" as above add 1 ml WVO
2. Mix thoroughly (it will turn yellow)
3. Add drop by drop the NaOH/KOH tester solution until you see a 30 second color change
see picture below sample D
(NOTE: Duller red colour than previous 30 second colour change due to WVO now being present)
4. Record the amount of NaOH/KOH tester solution you used
5. Repeat Steps 1 through 4 until you repeat your results two more times
Turmeric titration colour changes
- A = The prepared turmeric indicator solution ready for use
- B = A few drops of Sample A + 10ml of isopropyl alcohol, ready to do a blank titration
- C = Sample B + drops of NaOH/KOH tester solution added to get to required Ph switch point or blank titration
- D = Sample C + 1ml WVO + X drops of NaOH/KOH tester solution, note the dulling of the red colour due to the WVO.
PLEASE NOTE
Turmeric has a very strong yellow colouring and can seriously stain other materials it comes into contact with.
Turmeric titration colour changes