Difference between revisions of "Titrating with turmeric"
(UK spelling ... I hope!) |
|||
| (14 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| + | <metadesc>Biodiesel titration, Turmeric titration, Biodiesel titration instructions, Testing the pH level of vegetable oil, Indicator solution, Blank titration</metadesc> | ||
==Introduction== | ==Introduction== | ||
| − | How to prepare | + | How to prepare turmeric as a pH indicator solution, and use to titrate [[Waste vegetable oil]] to measure [[Free fatty acid (FFA)|Free fatty acid]] content. |
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! style="background: #F6E9EB;"|PLEASE NOTE | ||
| + | |- | ||
| + | |style="background: #F6E9EB;"|Turmeric has a very strong yellow colouring and can seriously stain materials it comes into contact with. | ||
| + | |} | ||
| − | |||
| − | |||
__TOC__ | __TOC__ | ||
| − | ==Why | + | ==Why we use indicators to titrate== |
| − | When titrating WVO to make biodiesel, we use an indicator solution to tell us when the FFAs have been | + | When titrating WVO to make biodiesel, we use an indicator solution to tell us when the FFAs have been neutralised. Experience has shown that pH meters don't work with wvo and are no substitute for a simple titration using indicator solution. |
| − | ==All | + | ==All about indicators== |
| − | Indicators are substances that change | + | Indicators are substances that change colour at a certain pH. All indicator solutions are made by adding a little bit of an indicator to a lot of alcohol. They do not change colour until the solution they are in becomes acidic or alkaline. The three most common indicators used for making biodiesel are phenolphthalein, phenol red, and turmeric. Phenolphthalein and turmeric change colour at the correct pH but phenol red does not. |
| − | '''Phenolphthalein''' this is the industry standard for titrating fats and oils to determine the percentage of FFAs they contain. It must be handled with care because it is | + | '''Phenolphthalein''' this is the industry standard for titrating fats and oils to determine the percentage of FFAs they contain. It must be handled with care because it is an irritant. Phenolphthalein changes from colourless to pink. |
'''Turmeric''' is by no means new as an indicator and references to it can be found in chemistry texts. Because turmeric is readily available in the spice section of many grocery stores and lacks the toxicity of phenolphthalein, its use as an indicator is on the rise. | '''Turmeric''' is by no means new as an indicator and references to it can be found in chemistry texts. Because turmeric is readily available in the spice section of many grocery stores and lacks the toxicity of phenolphthalein, its use as an indicator is on the rise. | ||
| − | Turmeric changes | + | Turmeric changes colour from yellow to orange/red at a pH close to that of phenolphthalein. |
| − | ==What | + | ==What is a "blank titration" and why do I care?== |
| − | The alcohol used to make indicator solutions may itself be acidic. This may also be true of the isopropyl alcohol used as a solvent in the titration itself. So it is common practice to do a "Blank Titration" to | + | The alcohol used to make indicator solutions may itself be acidic. This may also be true of the isopropyl alcohol used as a solvent in the titration itself. So it is common practice to do a "Blank Titration" to neutralise any acid which might be in the alcohol itself. |
| − | When you perform a "Blank Titration" you add titration indicator solution to alcohol and then add NaOH/KOH tester solution one drop at a time. Once the indicator just starts to turn | + | When you perform a "Blank Titration" you add titration indicator solution to alcohol and then add NaOH/KOH tester solution one drop at a time. Once the indicator just starts to turn colour in the blank titration you know all of the acid in the alcohol has been neutralised or eliminated. |
| − | After you | + | After you neutralise the acid in the alcohol, you know that the titration will measure just the acid in the oil itself. Following a "Blank Titration", you can simply add the oil to the neutralised alcohol and proceed to perform the titration itself. |
| − | Turmeric indicator solution starts to change | + | Turmeric indicator solution starts to change colour between pH 7.4 (above neutral) and pH 8.5 (alkaline). For our purposes as biodiesel homebrewers, this is accurate enough. With some alcohol, adding just one or two drops of NaOH/KOH tester solution to a "Blank Titration" may achieve a colour change but with other alcohol it may take seven or eight drops. |
| − | ==What | + | ==What does it mean to "neutralise" free fatty acids (FFAs) to pH 8.5== |
When we titrate oil in solution with alcohol, we conduct a chemical reaction between the FFAs in the oil and the NaOH or KOH in the solution. This chemical reaction makes soap, which is alkaline. | When we titrate oil in solution with alcohol, we conduct a chemical reaction between the FFAs in the oil and the NaOH or KOH in the solution. This chemical reaction makes soap, which is alkaline. | ||
| Line 40: | Line 46: | ||
IS the same shade when you're doing a "Blank Titration" (which will be transparent) AND when you're titrating oil (which will be duller or translucent) | IS the same shade when you're doing a "Blank Titration" (which will be transparent) AND when you're titrating oil (which will be duller or translucent) | ||
| − | So if one person's turmeric indicator starts yellow and changes into an orange-rose and someone else's starts greenish-yellow and changes into orange or red (as in this example picture at the bottom of the page), it doesn't matter. What matters is that the | + | So if one person's turmeric indicator starts yellow and changes into an orange-rose and someone else's starts greenish-yellow and changes into orange or red (as in this example picture at the bottom of the page), it doesn't matter. What matters is that the colour has changed from the original yellow colour to the same colour as your blank titration and stayed that way for 30 seconds, do some tests with your turmeric and observe the shade or colour it actually changes to when blanked. |
| − | This means that you shouldn't "push" a turmeric or phenolphthalein titration to yield a particular shade or intensity of | + | This means that you shouldn't "push" a turmeric or phenolphthalein titration to yield a particular shade or intensity of colour. As soon as the color changes and persists for 30 seconds, the titration is complete. The key is to use a consistent approach from titration to titration, it should be noted that when titrating with turmeric the titration mixture has a cloudy look to it and is not clear like phenolpthalien. |
| − | == | + | ==Preparation== |
| − | ===Reference | + | ===Reference solution=== |
First, make a 0.1% NaOH/KOH [[Titration#Reference_solution|reference solution]] - 1 gram of catalyst (NaOH or KOH) dissolved in 1 litre of distilled water. | First, make a 0.1% NaOH/KOH [[Titration#Reference_solution|reference solution]] - 1 gram of catalyst (NaOH or KOH) dissolved in 1 litre of distilled water. | ||
===Turmeric indicator solution=== | ===Turmeric indicator solution=== | ||
| Line 54: | Line 60: | ||
==Titration== | ==Titration== | ||
| − | ===Blanking the | + | ===Blanking the isopropyl alcohol=== |
| − | # Measure 10 ml | + | # Measure 10 ml isopropyl alcohol into your titration vessel. |
# Add several drops of turmeric indicator solution until the alcohol turns yellow. See [[#Colour changes|sample B]]. | # Add several drops of turmeric indicator solution until the alcohol turns yellow. See [[#Colour changes|sample B]]. | ||
# Add just enough drops of NaOH/KOH tester solution until you see a 30 second red colour change. See [[#Colour changes|sample C]]. | # Add just enough drops of NaOH/KOH tester solution until you see a 30 second red colour change. See [[#Colour changes|sample C]]. | ||
| − | ===Titrating the | + | ===Titrating the waste vegetable oil=== |
| − | # Add 1 ml WVO to the 10 ml of Isopropyl Alcohol that you have | + | # Add 1 ml WVO to the 10 ml of Isopropyl Alcohol that you have neutralised in a "Blank Titration" as above. |
# Mix thoroughly (it will turn yellow). | # Mix thoroughly (it will turn yellow). | ||
# Add drop by drop the NaOH/KOH tester solution until you see a 30 second colour change. See [[#Colour changes|sample D]], noting duller red colour than previous 30 second colour change due to the presence of WVO. | # Add drop by drop the NaOH/KOH tester solution until you see a 30 second colour change. See [[#Colour changes|sample D]], noting duller red colour than previous 30 second colour change due to the presence of WVO. | ||
| Line 76: | Line 82: | ||
* D = Sample C + 1ml WVO + X drops of NaOH/KOH tester solution, note the dulling of the red colour due to the WVO. | * D = Sample C + 1ml WVO + X drops of NaOH/KOH tester solution, note the dulling of the red colour due to the WVO. | ||
| − | [[ | + | |
| + | ==See also== | ||
| + | |||
| + | * [[MSDS - 2-propanol]] | ||
| + | * [[MSDS - Phenolphthalein]] | ||
| + | |||
| + | |||
[[Category:Biodiesel]] | [[Category:Biodiesel]] | ||
| + | [[Category:Ingredients and preparation]] | ||
Latest revision as of 13:39, 24 February 2013
Introduction
How to prepare turmeric as a pH indicator solution, and use to titrate Waste vegetable oil to measure Free fatty acid content.
| PLEASE NOTE |
|---|
| Turmeric has a very strong yellow colouring and can seriously stain materials it comes into contact with. |
Contents
Why we use indicators to titrate
When titrating WVO to make biodiesel, we use an indicator solution to tell us when the FFAs have been neutralised. Experience has shown that pH meters don't work with wvo and are no substitute for a simple titration using indicator solution.
All about indicators
Indicators are substances that change colour at a certain pH. All indicator solutions are made by adding a little bit of an indicator to a lot of alcohol. They do not change colour until the solution they are in becomes acidic or alkaline. The three most common indicators used for making biodiesel are phenolphthalein, phenol red, and turmeric. Phenolphthalein and turmeric change colour at the correct pH but phenol red does not.
Phenolphthalein this is the industry standard for titrating fats and oils to determine the percentage of FFAs they contain. It must be handled with care because it is an irritant. Phenolphthalein changes from colourless to pink.
Turmeric is by no means new as an indicator and references to it can be found in chemistry texts. Because turmeric is readily available in the spice section of many grocery stores and lacks the toxicity of phenolphthalein, its use as an indicator is on the rise. Turmeric changes colour from yellow to orange/red at a pH close to that of phenolphthalein.
What is a "blank titration" and why do I care?
The alcohol used to make indicator solutions may itself be acidic. This may also be true of the isopropyl alcohol used as a solvent in the titration itself. So it is common practice to do a "Blank Titration" to neutralise any acid which might be in the alcohol itself.
When you perform a "Blank Titration" you add titration indicator solution to alcohol and then add NaOH/KOH tester solution one drop at a time. Once the indicator just starts to turn colour in the blank titration you know all of the acid in the alcohol has been neutralised or eliminated.
After you neutralise the acid in the alcohol, you know that the titration will measure just the acid in the oil itself. Following a "Blank Titration", you can simply add the oil to the neutralised alcohol and proceed to perform the titration itself.
Turmeric indicator solution starts to change colour between pH 7.4 (above neutral) and pH 8.5 (alkaline). For our purposes as biodiesel homebrewers, this is accurate enough. With some alcohol, adding just one or two drops of NaOH/KOH tester solution to a "Blank Titration" may achieve a colour change but with other alcohol it may take seven or eight drops.
What does it mean to "neutralise" free fatty acids (FFAs) to pH 8.5
When we titrate oil in solution with alcohol, we conduct a chemical reaction between the FFAs in the oil and the NaOH or KOH in the solution. This chemical reaction makes soap, which is alkaline.
Chemists have figured out that when ALL the FFAs have been turned into soap, the pH of the solution will rise to pH 8.5. They refer to this process as neutralisation, as the FFAs (acids) have been eliminated. But keep in mind that this neutralized solution is alkaline (pH 8.5), NOT chemically neutral (pH 7).
Indicators that can tell us when a solution has a pH around pH 8.5 are most appropriate for biodiesel homebrewers. That is why turmeric and phenolphthalein are suitable, as they change colour at pH levels that are close enough to pH 8.5 for our purposes. Turmeric contains a yellow pigment called curcumin that is yellow at pH 7.4 and orange to red at pH 8.6. Phenolpthalein is colourless up to pH 8.3 when it turns pink, through to deep red at pH 9.
Chemists before us figured out what a titration's pH is supposed to be and the experiences of thousands of biodiesel homebrewers have confirmed this in practice. So if you use an indicator (pH strips, bromthymol blue, etc.) that requires you to compare the colour of your results against a scale, be sure that you titrate to pH 8.5 NOT to pH 7.
Indicator Colour Change Persists 30 Seconds During Titrations Whether you are performing a "Blank Titration" of alcohol or titrating oil in an alcohol solution, look for a colour change that persists for 30 seconds. The exact colour or shade is relatively unimportant so long as it: IS NOT the same colour as when you began the titration IS the same shade when you're doing a "Blank Titration" (which will be transparent) AND when you're titrating oil (which will be duller or translucent)
So if one person's turmeric indicator starts yellow and changes into an orange-rose and someone else's starts greenish-yellow and changes into orange or red (as in this example picture at the bottom of the page), it doesn't matter. What matters is that the colour has changed from the original yellow colour to the same colour as your blank titration and stayed that way for 30 seconds, do some tests with your turmeric and observe the shade or colour it actually changes to when blanked.
This means that you shouldn't "push" a turmeric or phenolphthalein titration to yield a particular shade or intensity of colour. As soon as the color changes and persists for 30 seconds, the titration is complete. The key is to use a consistent approach from titration to titration, it should be noted that when titrating with turmeric the titration mixture has a cloudy look to it and is not clear like phenolpthalien.
Preparation
Reference solution
First, make a 0.1% NaOH/KOH reference solution - 1 gram of catalyst (NaOH or KOH) dissolved in 1 litre of distilled water.
Turmeric indicator solution
- Thoroughly mix ground Turmeric with Isopropyl Alcohol using 1 part turmeric to 5 parts alcohol
- Let the mixture settle
- Decant the liquid into a small bottle with a dropper.
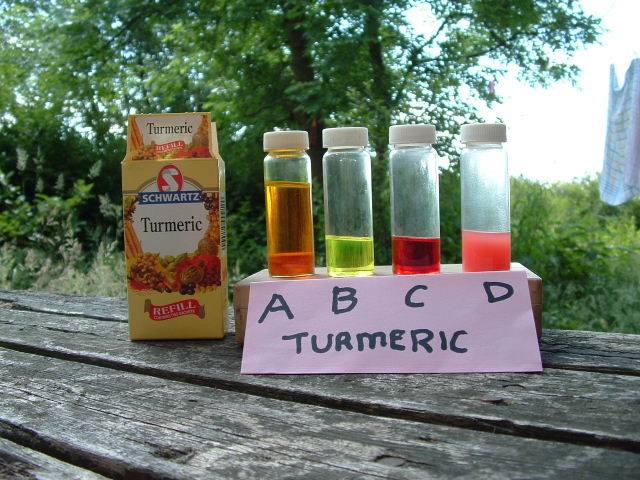
See sample A.
Titration
Blanking the isopropyl alcohol
- Measure 10 ml isopropyl alcohol into your titration vessel.
- Add several drops of turmeric indicator solution until the alcohol turns yellow. See sample B.
- Add just enough drops of NaOH/KOH tester solution until you see a 30 second red colour change. See sample C.
Titrating the waste vegetable oil
- Add 1 ml WVO to the 10 ml of Isopropyl Alcohol that you have neutralised in a "Blank Titration" as above.
- Mix thoroughly (it will turn yellow).
- Add drop by drop the NaOH/KOH tester solution until you see a 30 second colour change. See sample D, noting duller red colour than previous 30 second colour change due to the presence of WVO.
- Record the amount of NaOH/KOH tester solution you used
- Repeat Steps 1 through 4 until you repeat your results two more times
Turmeric titration colour changes
- A = The prepared turmeric indicator solution ready for use
- B = A few drops of Sample A + 10ml of Isopropyl Alcohol, ready to do a blank titration
- C = Sample B + drops of NaOH/KOH tester solution added to get to required pH switch point or blank titration
- D = Sample C + 1ml WVO + X drops of NaOH/KOH tester solution, note the dulling of the red colour due to the WVO.