Testing methanol purity
For best results the methanol used in biodiesel production needs to be a minimum of 97% pure. It’s good practise to test the purity of a new batch of methanol firstly to ensure you are getting what you paid for, not substandard product from an unscrupulous supplier and secondly to ensure that it’s not going to cause problems during production. As methanol is hydroscopic (able to absorb moisture from the atmosphere) is may be of benefit to test old stock or methanol that has been open to the atmosphere for any length of time. Perhaps the most beneficial use of SG tests is on methanol recovered from biodiesel to see if it’s suitable for use in the next batch.
Contents
Specific gravity
Pure methanol has an SG (specific gravity) of 0.7913 (0.79 for our purposes) at 20°C. For those unfamiliar with specific gravity, it’s a comparison of the density (mass per volume at a specific temperature and pressure) of a substance with the density of pure water. Water has a specific gravity of 1. So from the figures above it’s obvious that methanol is less dense that water. If methanol and water were not miscible (able to mix in all proportions, i.e. one is soluble in the other) and we poured some methanol into a beaker of water, it would float as a layer at the top. However, water and methanol are miscible and therefore cause us some problems in the production of biodiesel, water promoting the formation of soap during the process.
Testing with a hydrometer
Hydrometers
Many people may be familiar with a hydrometer, they are frequently used to test battery acid, for testing concentrations of anti-freeze in car engines and to check the salinity of salt water aquariums. More accurate versions, covering a limited range of SG are available for laboratory use and can be found at reasonable prices on internet auction sites. When purchasing this type of hydrometer, be sure that it covers a range suitable for testing methanol. Typically a laboratory hydrometer of this type will have a range between 0.750 - .850 with 0.0005 divisions.
Also suitable, but not quite so accurate, is a Trellis hydrometer used in home brewing. These often read in % alcohol, proof or ABV (alcohol by volume). They will be based on ethanol which comprises the bulk of alcohol found in common beverages. Ethanol has an SG of 0.789, so very close to methanol, not as accurate as a laboratory version, but good enough for our needs if economy is an overriding factor.
Hydrometer trail jar
In addition to the hydrometer, you will need what’s known as a trial or test jar. This is simply a tall, preferably transparent jar in which the hydrometer will float without touching the sides or bottom. A measuring cylinder is ideal, but these can be quite expensive in larger sizes.
A cheaper alternative can be constructed at home. The one pictured below was made in a few minutes using scrap materials. As the cylinder isn't transparent, it's necessary to fill it to the top in order to see the reading. In operation the tube is filled to the top and the hydrometer inserted. The liquid displaced is caught by the cup surrounding the tube.
- Home made hydrometer trail jar
As an alternative to the above method of construction, the base could be cast in lead. Lead could be melted into a suitably sized tin lid and a length of 28mm, or larger, copper or steel tube with the end tinned placed just below the surface of the molten lead and the assembly allowed to cool. The cup could be constructed in the same way as the photos above.
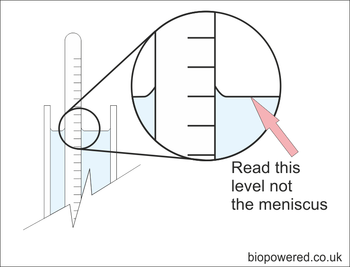
Reading a hydrometer
If you are using a transparent trial jar, fill it with sufficient methanol to allow the hydrometer to float well clear of the bottom. Give it a gentle spin to remove any air bubbles and allow it to settle. The SG can be read from the scale
The following table is reproduced with kind permission of Graham Laming:
Assuming water is the pollutant, this table gives you the relationship between density and purity ....
Methyl Alcohol (CH3OH) Density kg/litre
| % pure | 0°C | 10°C | 15°C | 20°C |
| 90 | 0.8374 | 0.8287 | 0.8239 | 0.8202 |
| 91 | 0.8347 | 0.8261 | 0.8212 | 0.8174 |
| 92 | 0.832 | 0.8234 | 0.8185 | 0.8146 |
| 93 | 0.8293 | 0.8208 | 0.8157 | 0.8118 |
| 94 | 0.8266 | 0.818 | 0.8129 | 0.809 |
| 95 | 0.824 | 0.8152 | 0.8101 | 0.8062 |
| 96 | 0.8212 | 0.8124 | 0.8073 | 0.8034 |
| 97 | 0.8186 | 0.8096 | 0.8045 | 0.8005 |
| 98 | 0.8158 | 0.8068 | 0.8016 | 0.7976 |
| 99 | 0.813 | 0.804 | 0.7987 | 0.7948 |
| 100 | 0.8102 | 0.8009 | 0.7959 | 0.7917 |
Testing by weight
So, as long as 1 litre weighs less than 800g at 20C, it should be OK to use directly. (Better than 97% pure)