Difference between revisions of "Catalyst"
(Add Catalyst to Biodiesel category) |
|||
| (16 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | <metadesc>Biodiesel catalysts, Sodium Hydroxide, NaOH, Potassium Hydroxide, KOH, Sodium Methylate, NaOCH3</metadesc> | |
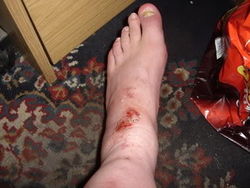
| − | Sodium Hydroxide | + | [[File:NaoH burn - e3msb.jpg|250px|thumb|right|Burns as a result of NaOH falling inside a rigger boot. Source: e3msb.]] |
| + | [[Sodium hydroxide]] and [[potassium hydroxide]] are popular catalysts for home biodiesel production. They are mixed with [[methanol]] to form a [[methoxide]]. | ||
| + | Alternatively, [[anhydrous sodium methylate]] may be diluted to the correct strength with methanol. | ||
| − | ==Sodium | + | |
| + | __TOC__ | ||
| + | |||
| + | |||
| + | |||
| + | ==Sodium hydroxide (NaOH)== | ||
* inexpensive | * inexpensive | ||
| − | * harder to dissolve into solution with | + | * harder to dissolve into solution with methanol |
| − | * solid [[ | + | * solid [[glycerol]] by-product |
* accurate titration required to avoid making excess soap | * accurate titration required to avoid making excess soap | ||
| + | * soaps will settle quicker than potassium | ||
| + | * come in pearls which are less likely to become airborne unlike flakes. | ||
| + | |||
| + | |||
| − | ==Potassium | + | ==Potassium hydroxide (KOH)== |
* more costly | * more costly | ||
| − | * easy to dissolve into solution with | + | * easy to dissolve into solution with methanol |
| − | * liquid | + | * come in flakes which can become airborne, ensure you use face protection. |
| + | * liquid glycerol by-product | ||
* forgiving with inaccurate titrations | * forgiving with inaccurate titrations | ||
| + | |||
| + | |||
| + | |||
| + | ==Anhydrous sodium methylate== | ||
| + | * around 4 x the cost of mixing (sodium hydroxide and methanol) | ||
| + | * pre-mixed catalyst in methanol (25%, 30%, 32% by weight) | ||
| + | * easily diluted to the correct strength in methanol | ||
| + | * no water present, therefore no soaps produced | ||
| + | * very dangerous, used for large scale production when the effect on yield make it cost effective | ||
| + | |||
| + | |||
==Storage and handling== | ==Storage and handling== | ||
| − | Both catalysts should be kept in a dry, sealed airtight container away from the reach of children. | + | Both potassium hydroxide and sodium hydroxide catalysts should be kept in a dry, sealed airtight container, If left exposed the catalyst will absorb moisture and carbon dioxide from the atmosphere and weaken the catalyst. Ensure it is kept away from animals and out of the reach of children. |
Protective gloves and eye equipment must be worn during handling, as both are strong bases and will dissolve flesh into soap. | Protective gloves and eye equipment must be worn during handling, as both are strong bases and will dissolve flesh into soap. | ||
Care should be taken with "flake" products to avoid inhalation. | Care should be taken with "flake" products to avoid inhalation. | ||
| + | |||
| + | |||
| + | |||
| + | ==See also== | ||
| + | |||
| + | * [[MSDS - Potassium Hydroxide ]] | ||
| + | * [[MSDS - Sodium Hydroxide ]] | ||
[[Category:Biodiesel]] | [[Category:Biodiesel]] | ||
| + | [[Category:Chemicals]] | ||
Latest revision as of 11:31, 10 March 2013
Sodium hydroxide and potassium hydroxide are popular catalysts for home biodiesel production. They are mixed with methanol to form a methoxide. Alternatively, anhydrous sodium methylate may be diluted to the correct strength with methanol.
Contents
Sodium hydroxide (NaOH)
- inexpensive
- harder to dissolve into solution with methanol
- solid glycerol by-product
- accurate titration required to avoid making excess soap
- soaps will settle quicker than potassium
- come in pearls which are less likely to become airborne unlike flakes.
Potassium hydroxide (KOH)
- more costly
- easy to dissolve into solution with methanol
- come in flakes which can become airborne, ensure you use face protection.
- liquid glycerol by-product
- forgiving with inaccurate titrations
Anhydrous sodium methylate
- around 4 x the cost of mixing (sodium hydroxide and methanol)
- pre-mixed catalyst in methanol (25%, 30%, 32% by weight)
- easily diluted to the correct strength in methanol
- no water present, therefore no soaps produced
- very dangerous, used for large scale production when the effect on yield make it cost effective
Storage and handling
Both potassium hydroxide and sodium hydroxide catalysts should be kept in a dry, sealed airtight container, If left exposed the catalyst will absorb moisture and carbon dioxide from the atmosphere and weaken the catalyst. Ensure it is kept away from animals and out of the reach of children.
Protective gloves and eye equipment must be worn during handling, as both are strong bases and will dissolve flesh into soap.
Care should be taken with "flake" products to avoid inhalation.