Difference between revisions of "Catalyst"
Julesandtash (talk | contribs) |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
<metadesc>Biodiesel catalysts, Sodium Hydroxide, NaOH, Potassium Hydroxide, KOH, Sodium Methylate, NaOCH3</metadesc> | <metadesc>Biodiesel catalysts, Sodium Hydroxide, NaOH, Potassium Hydroxide, KOH, Sodium Methylate, NaOCH3</metadesc> | ||
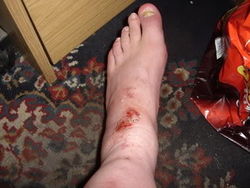
[[File:NaoH burn - e3msb.jpg|250px|thumb|right|Burns as a result of NaOH falling inside a rigger boot. Source: e3msb.]] | [[File:NaoH burn - e3msb.jpg|250px|thumb|right|Burns as a result of NaOH falling inside a rigger boot. Source: e3msb.]] | ||
| − | Sodium | + | [[Sodium hydroxide]] and [[potassium hydroxide]] are popular catalysts for home biodiesel production. They are mixed with [[methanol]] to form a [[methoxide]]. |
| − | Alternatively, | + | Alternatively, [[anhydrous sodium methylate]] may be diluted to the correct strength with methanol. |
__TOC__ | __TOC__ | ||
| − | ==Sodium | + | |
| + | |||
| + | ==Sodium hydroxide (NaOH)== | ||
* inexpensive | * inexpensive | ||
| − | * harder to dissolve into solution with | + | * harder to dissolve into solution with methanol |
| − | * solid [[ | + | * solid [[glycerol]] by-product |
* accurate titration required to avoid making excess soap | * accurate titration required to avoid making excess soap | ||
| − | * soaps will settle quicker than | + | * soaps will settle quicker than potassium |
* come in pearls which are less likely to become airborne unlike flakes. | * come in pearls which are less likely to become airborne unlike flakes. | ||
| − | ==Potassium | + | |
| + | |||
| + | ==Potassium hydroxide (KOH)== | ||
* more costly | * more costly | ||
| − | * easy to dissolve into solution with | + | * easy to dissolve into solution with methanol |
* come in flakes which can become airborne, ensure you use face protection. | * come in flakes which can become airborne, ensure you use face protection. | ||
| − | * liquid | + | * liquid glycerol by-product |
* forgiving with inaccurate titrations | * forgiving with inaccurate titrations | ||
| − | == | + | |
| − | * around 4 x the cost of mixing ( | + | |
| − | * pre-mixed catalyst in | + | ==Anhydrous sodium methylate== |
| − | * easily diluted to the correct strength in | + | * around 4 x the cost of mixing (sodium hydroxide and methanol) |
| + | * pre-mixed catalyst in methanol (25%, 30%, 32% by weight) | ||
| + | * easily diluted to the correct strength in methanol | ||
* no water present, therefore no soaps produced | * no water present, therefore no soaps produced | ||
* very dangerous, used for large scale production when the effect on yield make it cost effective | * very dangerous, used for large scale production when the effect on yield make it cost effective | ||
| + | |||
| + | |||
==Storage and handling== | ==Storage and handling== | ||
| − | Both | + | Both potassium hydroxide and sodium hydroxide catalysts should be kept in a dry, sealed airtight container, If left exposed the catalyst will absorb moisture and carbon dioxide from the atmosphere and weaken the catalyst. Ensure it is kept away from animals and out of the reach of children. |
Protective gloves and eye equipment must be worn during handling, as both are strong bases and will dissolve flesh into soap. | Protective gloves and eye equipment must be worn during handling, as both are strong bases and will dissolve flesh into soap. | ||
Care should be taken with "flake" products to avoid inhalation. | Care should be taken with "flake" products to avoid inhalation. | ||
| + | |||
| + | |||
==See also== | ==See also== | ||
Latest revision as of 10:31, 10 March 2013
Sodium hydroxide and potassium hydroxide are popular catalysts for home biodiesel production. They are mixed with methanol to form a methoxide. Alternatively, anhydrous sodium methylate may be diluted to the correct strength with methanol.
Contents
Sodium hydroxide (NaOH)
- inexpensive
- harder to dissolve into solution with methanol
- solid glycerol by-product
- accurate titration required to avoid making excess soap
- soaps will settle quicker than potassium
- come in pearls which are less likely to become airborne unlike flakes.
Potassium hydroxide (KOH)
- more costly
- easy to dissolve into solution with methanol
- come in flakes which can become airborne, ensure you use face protection.
- liquid glycerol by-product
- forgiving with inaccurate titrations
Anhydrous sodium methylate
- around 4 x the cost of mixing (sodium hydroxide and methanol)
- pre-mixed catalyst in methanol (25%, 30%, 32% by weight)
- easily diluted to the correct strength in methanol
- no water present, therefore no soaps produced
- very dangerous, used for large scale production when the effect on yield make it cost effective
Storage and handling
Both potassium hydroxide and sodium hydroxide catalysts should be kept in a dry, sealed airtight container, If left exposed the catalyst will absorb moisture and carbon dioxide from the atmosphere and weaken the catalyst. Ensure it is kept away from animals and out of the reach of children.
Protective gloves and eye equipment must be worn during handling, as both are strong bases and will dissolve flesh into soap.
Care should be taken with "flake" products to avoid inhalation.